Focusing on Innovation and 505b(2) , 6th PharmaCon 2020 is coming to Shanghai in October!
In October, Jointly hosted by the China Pharmaceutical Industry Association and BIOMAP, supported by CBA, PharmaCon 2020(the 6th World-China Pharmaceutical R&D Congress) will be held at InterContinental Jing’an hotel in Shanghai. More than 40 new drug research and development leading companies, regulatory experts, and scientists will be invited to focus on Type 1 innovation drugs and Type 2 modified drugs(505b(2)), analyzing a series of new drug regulations under the new version of the drug administration measures. PharmaCon 2020 will also discuss the cutting-edge innovative research and development of new-target pharmaceuticals, and share the leading practice of industrialization of high-end formulations, technology, quality control, and evaluation. In this case, the transformation and upgrading of R&D of new drugs with Chinese characteristics will be further facilitated.
Call for speaker! If you are expert on innovative chemical drug discovery, 505b(2), new formulation or CMC with successful IND/NDA experience, pls join us as PharmaCon honorable speaker online or onsite!
The booth of PharmaCon 2020 is in hot reservation! Join PharmaCon 2020 in form of keynote speech, product demonstration, cooperative invitation and so much more for you to show your innovative programs for R&D of new drugs! Limited booth, unlimited business opportunities! First come, First served, please contact us for reservation! 24h reservation hotline/wechat:+86 18017939885
(PharmaCon2020)The first batch of forum guests:
- Pan Guangcheng, Chairman, China Pharmaceutical Industry Association;
- Chemical Department of China Academy of food and drug control;
- Meicheng Yang, deputy director of Shanghai Institute for drug control;
- Ao Zhang, Dean of School of pharmacy, Shanghai Jiaotong University;
- Sanming Li, Professor of Shenyang Pharmaceutical University, chief scientist of Luoxin Pharmaceutical Group;
- Zeren Wang, chairman and chief scientific officer of HLK Pharma, Acting Vice President of Boehringer-Ingelheim’s former preparation department;
- Hong Chen, deputy general manager of Chengdu Easton Pharma and general manager of R & D Center
- Hequn Yin, chief scientific officer of Qilu pharmaceutical;
- Yun Lu, President of Hengrui Medicine Research Institute;
- Yusheng Wu, chairman of TYK Medicines Co., Ltd;
- Xinghai Li, co founder / chief technology officer of Haichuang Pharmaceutical Co., Ltd;
- Wei Long, vice president of R & D of Jacobio Pharma;
- Binhua Lv, deputy general manager of Zelgen;
- Bin Shi, Chief Strategic Officer of TYK Medicines Co., Ltd;
- Zhixuan Wang, CMC director of Sanofi;
- Shangjun Teng, senior director of Ascentage Pharma;
- Enxian Lu, general manager of Auson Pharma, former Novartis preparation expert;
- Feihuang Deng, technical director of Nanjing TRIASTEK Pharmaceutical | 3D Technology Co., Ltd;

More speakers are being updated……
Highlights and topics of the Forum (Partial):
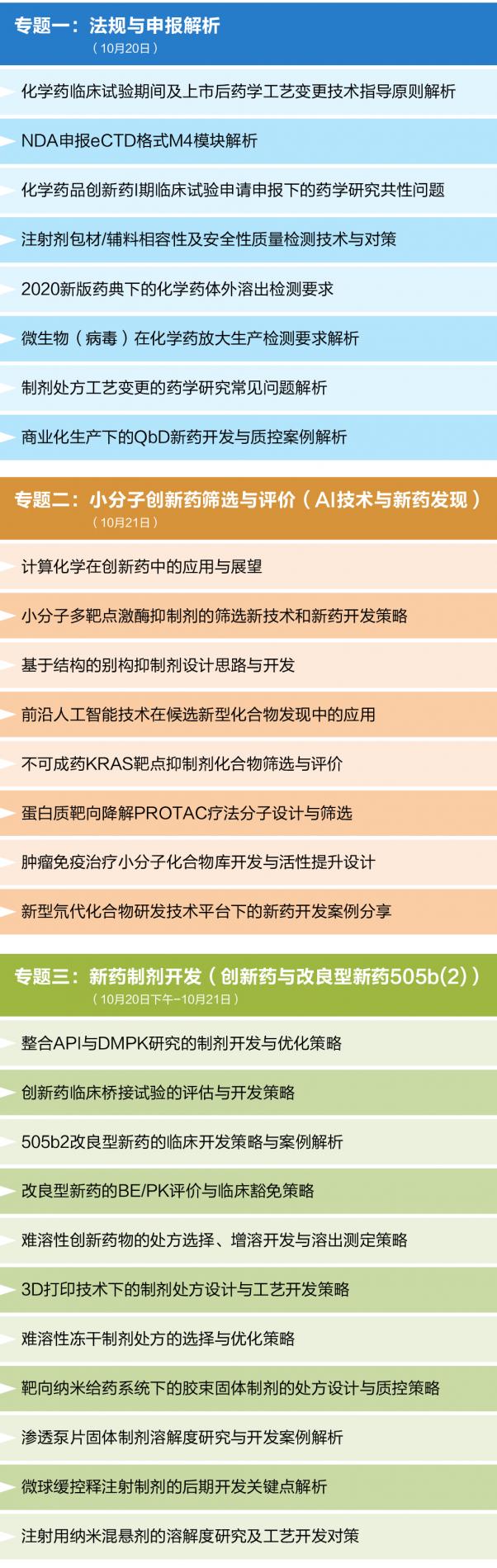
Session 1: Analysis of Drug Regulations and Declaration(October 20th)
- Analysis of Technical Guidelines for Changes in Pharmaceutical Process During Clinical Trials and Commercialization of Drugs
- Analysis of M4 Module in eCTD format during NDA Declaration
- Common Issues of Pharmaceutical Research under the Application for Phase I Clinical Trial of Innovative Pharmaceuticals
- Compatibility, Safety and Quality Inspection Techniques and Countermeasures of Injection Packaging Materials/Excipients
- Requirements for In-vitro Dissolution Testing of Pharmaceuticals Under the New Pharmacopoeia
- Research and Control Strategy of Drug Genotoxicity/Element Impurities During Application
- Analysis of Common Problems in Pharmacy Research on Changes in Formulations
- Case Study of QbD New Drug Development and Quality Control for Commercialization
Session 2: Small Molecule Innovative Drug Screening and Evaluation (Artificial Intelligence & New Drug Discovery)(October 21th)
- Application and Prospect of Computational Chemistry in Innovative Drugs
- New Screening Techniques and Drug Development Strategies for Small Molecule Multi-target Kinase Inhibitors
- Design and Development of Allosteric Inhibitors Based on Structure
- Application of Cutting-edge Artificial Intelligence in the Discovery of Candidate New Compounds
- Screening and Evaluation of Non-druggable KRAS Target Inhibitor Compounds
- Molecular Design and Screening of Protein Targeted Degradation PROTAC Therapy
- Development of Small Molecule Compound Library and Activity Enhancement Design for Tumor Immunotherapy
- Case Study of New Drug Development Based on the New R&D Approach of New Deuterated Compound
Session 3: New Formulation Development (Innovative Drug and Modified New Drug 505b (2))(October 20 afternoon – October 21)
- Formulation Development and Optimization Strategies Based on API and DMPK Research
- Evaluation and Development Strategies for Clinical Bridging Trials of Innovative Drugs
- Clinical Development Strategy and Case Study of 505b2 Modified New Drugs
- BE/PK Evaluation and Clinical Exemption Strategies for Modified New Drugs
- Prescription Selection, Solubilization Development and Dissolution Testing Strategy of Insoluble Innovative Drugs
- Preparation Formulation Design and Process Development Strategy via 3D Printing
- Selection and Optimization Strategy of Insoluble Freeze-dried Formulations
- Formulation Design and Quality Control Strategy of Micellar Solid Preparations under Targeted Nano Drug Delivery System
- Case Study of R&D on the Solubility of Solid Preparations of Osmotic Pump Tablets
- Key Points in the Late-Stage Development of Microsphere Sustained and Controlled Release Injection Preparations
- Study on the Solubility of Nano-suspensions for Injection and Process Development Strategies
For more highlights, please contact the Organizing Committee for details!
Early bird activity is about to end!
Special offer for the group: Buy 3 tickets and you can get 1 free (with equal rights)!
A special discount of 10% will be offered for single ticket purchase. You can enjoy the discount by using the discount code drth on the official website!
Scan the code to register and get the discount tickets

Contact Us:
Tel:18017939885
Web:www.bmapglobal.com/pharmacon2020
聚焦创新药&改良型新药,第六届中国国际化学药研发论坛金秋10月上海重磅重启

金秋10月,中国化学制药工业协会与商图信息联合主办,美国华人生物医药科技协会CBA支持, “PharmaCon 2020 第六届中国国际化学药研发论坛”将在上海静安洲际酒店盛大回归,本届论坛甄选40余位新药研发领军企业、法规监管专家与科学家,将围绕1类创新药(protac、氘代、激酶抑制剂、IO等)、2类改良型新药/505b(2)等品类,解析新版药品管理办法下的一系列化学药新规,探讨新型靶点化学药筛选的前沿创新研发,分享高端制剂处方、工艺与质控与评价的产业化领先实践,进而探索中国特色的新药转型升级的研发之路!
PharmaCon 2020火热招商中!论坛开放主题演讲、产品展示、合作邀约等多种形式、全方位供您展示新药研发创新方案!展位有限,商机无限!优秀展位,先到先得,抓紧联系我们预定吧!24h预定热线:18017939885
PharmaCon2020论坛首批嘉宾抢鲜看:

更多嘉宾信息持续更新中……
论坛亮点议题设置(部分)
更多亮点设置可联系组委会咨询详情!
早鸟活动即将截止!
团定特惠:满3位另赠1张同等权益参会票!
单人购票限时特惠9折,进入官网使用优惠码drth享受减免折扣!
扫码进入官网注册领取优惠门票

联系我们:
组委会电话:18017939885

