维立志博于2021 ASCO年会上公布其抗LAG-3抗体LBL-007临床I期研究数据
维立志博生物科技 2021-06-04
2021年6月4日,南京维立志博宣布由其自主研发的抗LAG-3全人抗体LBL-007注射液治疗晚期实体瘤研究已完成I期临床。该研究是一项评价晚期实体瘤安全性、耐受性、药代动力学及有效性的I期临床研究。LBL-007注射液临床I期研究入选2021年美国临床肿瘤学会(ASCO),相关的临床数据将于6月4至8日在ASCO年会上公布。
LBL-007是一种由维立志博自主研发的IgG4亚型全人源单克隆抗体。通过结合人LAG-3蛋白,进一步阻断LAG-3蛋白与其配体的结合,从而解除LAG-3对T细胞的抑制作用,使T细胞恢复免疫功能,起到对肿瘤生长的抑制作用。LBL-007于2019年8月13日获批开展晚期实体瘤及淋巴瘤的一期临床试验,并于2020年3月27日通过美国FDA临床试验许可。2021年2月4日LBL-007联合君实生物的抗PD-1单抗药物特瑞普利单抗注射液治疗不可切除或转移性黑色素瘤患者的临床试验已在北京大学肿瘤医院完成首例患者给药。
纽福斯和霍德生物宣布达成战略合作,共同开发基于iPSC的眼科疾病细胞治疗
霍德生物 2021-06-01
国内首家眼科AAV基因治疗生物医药公司,纽福斯生物科技有限公司(下称“纽福斯“),及一家拥有领先的人诱导多能干细胞(iPSC)及神经细胞分化技术平台的生物医药公司,霍德生物工程有限公司(下称“霍德生物”)宣布达成战略合作伙伴关系,共同开发治疗眼部疾病的人iPSC衍生细胞产品。
此次合作结合了纽福斯在全球基因/细胞治疗药物开发的经验,对眼部疾病的深刻理解,以及霍德生物在人iPSC衍生临床细胞产品GMP生产和质量体系上的优势,有望提供新一代的眼部疾病治疗方法。基于此次的合作,霍德生物将获得用于开发视网膜退行性疾病的候选细胞产品的预付款和里程碑付款,纽福斯则有权获得该候选产品的独家授权,并负责产品的临床开发及商业化。此外,纽福斯将得到霍德生物的人iPSC重编程技术专利和GMP人iPSC细胞株的相应授权,并在产品开发的不同阶段向霍德支付相应的授权费用。同时,产品上市后霍德生物将有权享有一定的产品销售提成。
达冕生物(RNAimmune)完成$1000万美元种子轮融资 用以推动其mRNA疫苗和治疗药物研发创制
圣诺制药 2021-04-21
达冕生物(RNAimmune)是一家专门研究和开发mRNA疫苗和药物的生物医药技术企业。该公司今天宣布已完成$1000万美元种子轮(A前轮)融资。本轮融资由香港顺河(Smooth River)和香港鸿润(Hong Kong Hongrun)领投、上海沃嘉(Shanghai Walga)生物技术、高森林投资(High Forest Investment)和美国宏瓴西格玛(Terra Magnum Sigma)共同参与投资。美国圣诺制药(Sirnaomics Inc.) 进一步加大了对达冕的投资。
达冕生物是圣诺制药公司的分拆实体。自去年初成立以来,该公司已经建立和完善了用于mRNA疫苗和新药创制的一整套技术平台,专注在病毒传染病,肿瘤和罕见病的领域的临床急需。公司正在为新型mRNA新冠疫苗(针对南非病毒株等变异株)的临床申报(IND)进行准备工作,预计在本年内向美国FDA 申报。达冕生物积极推进具有自主知识产权的RAS肿瘤疫苗的项目,并通过与加州大学洛杉矶分校合作加速该项目的临床前药效学验证。公司目前的总部位于首都华盛顿近郊的马里兰,并已经在中国广州国际生物岛建立了研发中心。达冕公司在去年8月份完成了$235万美元的首轮种子融资。
达冕董事会董事长陆阳博士表示: “我们很高兴看到达冕生物的快速增长,并在这么短的时间内完成了两轮融资。最近Moderna和BioNtech对抗新冠病毒的mRNA疫苗取得的成功使我们坚信,用于开发新型治疗和疫苗的mRNA技术正在极具颠覆性地改变制药业的格局,也给我们带来了巨大的机遇。因此,达冕生物将继续培育和加强我们的全球创新努力,开发针对Covid-19和其他病毒感染的新疫苗,探索新的治疗方法与新肿瘤抗原方法或其他mRNA药物
百力司康B轮融资超4亿元,聚焦创新ADC
动脉网 2021-04-16
百力司康生物医药(杭州)有限公司(以下简称“百力司康”)宣布完成超过4亿元人民币B轮融资。本轮融资由高瓴创投领投,由Cormorant Asset Management、鼎珮集团(VMS Group)、和达生物医药基金共同参与投资,现有股东夏尔巴资本和东方富海持续加注。易凯资本在本次交易中担任百力司康的独家财务顾问。
本轮融资将用于BB-1701项目的国际多中心临床研究推进、产品线其他品种的临床前研究及注册申报和公司的研发生产基地建设。BB-1701是一种新型抗体偶联物(antibody-drug conjugate, ADC),目前正在进行中美联合一期临床研究,其毒素艾立布林(Eribulin)是从日本卫材引进的已获批上市的抗肿瘤小分子药,拥有临床验证的安全性和有效性。百力司康拥有该款ADC的开发和商业化的全球权益。公司第二款艾立布林ADC BB-1705项目预计将于今年中美双报。同时,公司也开发了具有自主知识产权的独特的定点偶联技术平台,正在积极推进梯度管线中多个best-in-class和first-in-class潜力产品的研发。
百力司康共同创始人、董事长及CEO魏紫萍博士表示:“我们非常荣幸能够获得生物医药领域顶尖投资机构的认可,感谢业界知名投资基金的鼎力支持以及现有股东的持续加持,也感谢我们落户的钱塘新区以及国内外合作伙伴在各方面的配合。希望在本轮融资的加速推动下,百力司康结合自主研发和外部合作高效地推进多个在研产品的开发和产业化,解决未满足的临床需求。”
Have You Heard Of The Chinese Biopharmaceutical Association (CBA)?
The Chinese Biopharmaceutical Association (CBA) is one of the largest Chinese American professional associations in the U.S. (www.cba-usa.org). It was founded in 1995 by Guo-Liang Yu, Ph.D., who today is the cofounder, chairman, and CEO of Apollomics, an oncology therapeutic discovery and development company (to be featured in an upcoming issue of Life Science Leader).


Introduction to Workshop Build A Strong Career Development – April 29, 2023
The first event notice from the CBA-GP Chinese Biopharmaceutical Association of Greater Philadelphia, a branch of CBA-GP, has arrived. We have carefully prepared a flagship event for job seekers, entrepreneurs, and researchers interested in commercialization. April...

Introduction to NIH Small Business Grants – Zoom Webinar – May 3, 2023
The first event notice from the CBA-GP Chinese Biopharmaceutical Association of Greater Philadelphia, a branch of CBA-GP, has arrived. We have carefully prepared a flagship event for job seekers, entrepreneurs, and researchers interested in commercialization. May 3rd...

CBA Greater Philadelphia Chapter Opening Ceremony and Innovation Entrepreneurship Forum
The Chinese Biopharmaceutical Association, USA - Greater Philadelphia (CBA-GP) held its opening ceremony and innovation entrepreneurship forum on February 4th in the suburbs of Philadelphia. CBA is one of the largest professional technical associations between China...
2022 CBA Appreciation Dinner圆满结束
2022年5月28日,美国华人生物医药科技协会(Chinese Biopharmaceutical Association, CBA)在Normandie Farm Restaurant成功举办了 ”2022 CBA Appreciation Dinner” 。 本次活动是自新冠疫情以来CBA的第一次大型线下聚会。宴会邀请了政府嘉宾、CBA董事会成员、CBA执行团队、志愿者、企业会员、合作公司和赞助公司,共有超过120人注册参加了此次聚会。还有不少朋友千里迢迢从外州赶来聚会,CBA新老朋友们在一起相谈甚欢,其乐融融!...
2022 CBA 职业发展论坛暨现场招聘会成功举办
2022年5月7日,一年一度期待已久的美国华人生物医药科技协会(Chinese Biopharmaceutical Association,CBA)职业发展论坛暨现场招聘会在Montgomery College, Rockville Campus, Science Center成功举行,圆满落下帷幕。 本次活动主题是“生物医药行业求职和准备”,采取了线下和线上同步直播的方式举行。活动得到了诸多生物医药公司的大力支持,有超过...
CBA/CCRA 3D Printing in Pharmaceutical Development Seminar Concluded Successfully
May, 22, Sunday, CBA and its CCRA group lit on the summer heat with a hotter-than-ever webinar on 3D Printings in Pharmaceutical Development: History, Current Landscape, and the Future. This webinar brought together the leading experts and forerunners on...

CBA2021马里兰年会

2021CBA马里兰年会

2021 CBA ANNUAL CONFERENCE

CBA Boston Chapter Kick-off event-Innovation 2021: Lessons learn in the pandemic and opportunities afterwards
Date: Feb 20, 2021 Time: 8:45 am -1:00 pm (EST) Register Now and Find the Connection Registration Link: http://bit.ly/2NQzET Agenda: Time Topic Speaker 8:45am-9:00am CBA-USA Boston Chapter Kick-off Ru Zheng 9:00am-9:30am Challenge and Opportunity in US-China...
亚盛医药杨大俊:中国创新药企业的征途是全球市场
2020年对于所有人都是异常艰辛与难忘的一年,我们见证历史,我们也改写历史。

Clinical Development and Innovation Forum 2021
临床开发与创新合作论坛 2021
Mar.10-11, 2021
Crown Plaza Shanghai NOAH Square, PR. China
Program Overview
China and Asia over the decades has been playing an increasingly important role in the context of clinical research activities due to the significant scientific investment by multinational companies as well as rapidly booming China domestic R&D programs. In past few years, the China regulatory agency has also issued several regulations and initiatives to accelerate the review and approval and improve the quality of new drugs and medical devices. With the joining of ICH in 2017, the government is continuing to improve the regulatory standards and systems to promote innovation, enhance the R&D ecosystem. However, the drug development is fraught with all kinds of challenges which needs innovation, efficiency and collaboration. In
return, the Clinical Development and Innovation Forum will gather leaders and scientists across pharma, biotech and academia for discussions and cases studies on the major topics including: Robust Clinical and Regulatory Strategy, Clinical Biomarkers and CDx, ImmunoOncology and Cell & Gene Therapies, Rare Disease, Data Sciences and Digital Innovation, AI and Machine Learning, China Domestic R&D Innovation, Clinical Operation and Patient Strategies etc, towards the Efficientand GCP-compliant Drug Development in the era of Precision Medicine.

Review
mRNA Vaccine—A Pioneer of COVID-19 Pandemic Termination
Miao Tan1,2, Li Li1,3, Tom Tang1, Haitao Yang1,4, Lei He1, Qiang Hu1,5*
1 Clinical and Regulatory Study Group. Chinese Biopharmaceutical Association, Potomac, MD, USA
2 Pfizer Inc., La Jolla, CA, USA
3 BLA Regulatory, LLC, Gaithersburg, MD. USA.
4 Shenzhen Institute for the Research, Sun Yat-sen University, Shenzhen, China
5 Hunter Medical LLC. Potomac, MD, USA
*Correspondence:
Qiang Hu, M.D. Executive Chairman, Hunter Medical LLC. Potomac, MD, USA
Email: patrickhumd@yahoo.com.
ABSTRACT
Vaccines are one of the major success stories of modern medicine. The development of vaccines progressed at a fairly slow rate until the last decade when new scientific discoveries and technologies led to innovative genebased vaccines. Gene-based vaccines are a completely new type of vaccine that are faster and cheaper to produce than traditional vaccines. mRNA vaccines use a different approach that takes advantage of processes
that are more efficient, cost-effective and safe. On the basis of these remarkable properties, mRNA vaccines quickly moved forward and within ten years were being used in some early clinical trials for infectious diseases and several types of cancer. The COVID-19 outbreak dramatically accelerated mRNA vaccines, moving them from development to authorized use in a record-setting ten months. In this review, we provide an overview of mRNA vaccine development and its application against the COVID-19 pandemic. As the first approved COVID vaccines, mRNA vaccines have been shown to be safe and mRNA technology will have a tremendous impact, not only on the pandemic, but also on the future treatment of many diseases [Am J Transl Med 2021.
5 (1): 13-24].
Two Immigrants, One Unique Plan For A Biopharma

HONG KONG, Feb 2, 2021 – (ACN Newswire) – The American Dream is why the United States is considered the “land of opportunity,” and why for centuries immigrants have flocked to our shores seeking their fortunes. Guo-Liang Yu, Ph.D., (China), and Sanjeev Redkar, Ph.D., (India), came to the U.S. in the 1980s. By anyone’s reasonable measure, both have achieved the American Dream. However, their dream as scientists remained at arm’s length. Their desire? Create a pharmaceutical company that leverages the best of what the U.S. and China have to offer toward discovering and developing new oncology therapeutics, while using the smallest drug development program possible to do so. This is the story of how the scientific careers of an intrapreneur (Redkar) and an entrepreneur (Yu) came together to cofound and co-lead Apollomics.
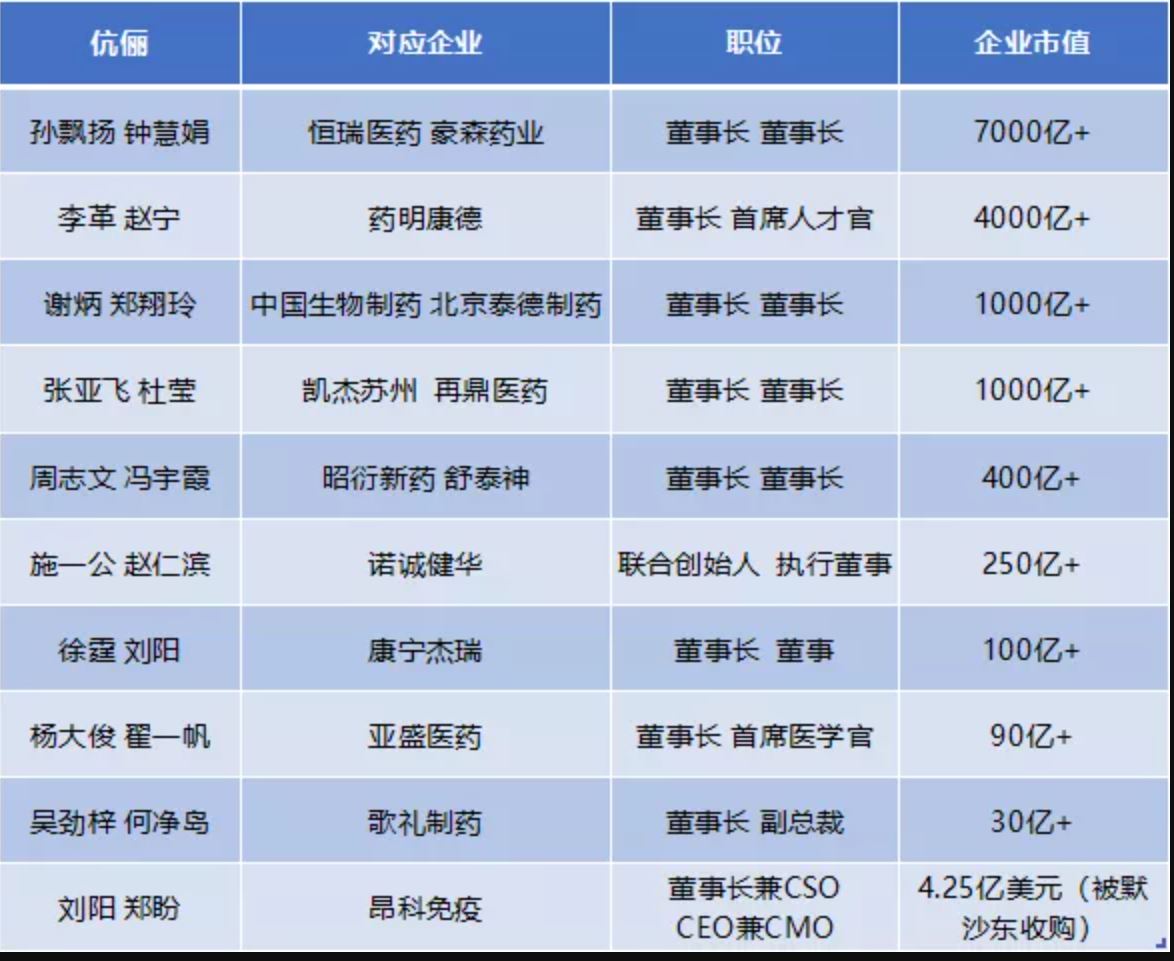
医药圈10对知名创业眷侣
康希诺生物-B(06185.HK)重组新型冠状病毒疫苗于墨西哥获得紧急使用授权











