维立志博于2021 ASCO年会上公布其抗LAG-3抗体LBL-007临床I期研究数据
维立志博生物科技 2021-06-04
2021年6月4日,南京维立志博宣布由其自主研发的抗LAG-3全人抗体LBL-007注射液治疗晚期实体瘤研究已完成I期临床。该研究是一项评价晚期实体瘤安全性、耐受性、药代动力学及有效性的I期临床研究。LBL-007注射液临床I期研究入选2021年美国临床肿瘤学会(ASCO),相关的临床数据将于6月4至8日在ASCO年会上公布。
LBL-007是一种由维立志博自主研发的IgG4亚型全人源单克隆抗体。通过结合人LAG-3蛋白,进一步阻断LAG-3蛋白与其配体的结合,从而解除LAG-3对T细胞的抑制作用,使T细胞恢复免疫功能,起到对肿瘤生长的抑制作用。LBL-007于2019年8月13日获批开展晚期实体瘤及淋巴瘤的一期临床试验,并于2020年3月27日通过美国FDA临床试验许可。2021年2月4日LBL-007联合君实生物的抗PD-1单抗药物特瑞普利单抗注射液治疗不可切除或转移性黑色素瘤患者的临床试验已在北京大学肿瘤医院完成首例患者给药。
纽福斯和霍德生物宣布达成战略合作,共同开发基于iPSC的眼科疾病细胞治疗
霍德生物 2021-06-01
国内首家眼科AAV基因治疗生物医药公司,纽福斯生物科技有限公司(下称“纽福斯“),及一家拥有领先的人诱导多能干细胞(iPSC)及神经细胞分化技术平台的生物医药公司,霍德生物工程有限公司(下称“霍德生物”)宣布达成战略合作伙伴关系,共同开发治疗眼部疾病的人iPSC衍生细胞产品。
此次合作结合了纽福斯在全球基因/细胞治疗药物开发的经验,对眼部疾病的深刻理解,以及霍德生物在人iPSC衍生临床细胞产品GMP生产和质量体系上的优势,有望提供新一代的眼部疾病治疗方法。基于此次的合作,霍德生物将获得用于开发视网膜退行性疾病的候选细胞产品的预付款和里程碑付款,纽福斯则有权获得该候选产品的独家授权,并负责产品的临床开发及商业化。此外,纽福斯将得到霍德生物的人iPSC重编程技术专利和GMP人iPSC细胞株的相应授权,并在产品开发的不同阶段向霍德支付相应的授权费用。同时,产品上市后霍德生物将有权享有一定的产品销售提成。
达冕生物(RNAimmune)完成$1000万美元种子轮融资 用以推动其mRNA疫苗和治疗药物研发创制
圣诺制药 2021-04-21
达冕生物(RNAimmune)是一家专门研究和开发mRNA疫苗和药物的生物医药技术企业。该公司今天宣布已完成$1000万美元种子轮(A前轮)融资。本轮融资由香港顺河(Smooth River)和香港鸿润(Hong Kong Hongrun)领投、上海沃嘉(Shanghai Walga)生物技术、高森林投资(High Forest Investment)和美国宏瓴西格玛(Terra Magnum Sigma)共同参与投资。美国圣诺制药(Sirnaomics Inc.) 进一步加大了对达冕的投资。
达冕生物是圣诺制药公司的分拆实体。自去年初成立以来,该公司已经建立和完善了用于mRNA疫苗和新药创制的一整套技术平台,专注在病毒传染病,肿瘤和罕见病的领域的临床急需。公司正在为新型mRNA新冠疫苗(针对南非病毒株等变异株)的临床申报(IND)进行准备工作,预计在本年内向美国FDA 申报。达冕生物积极推进具有自主知识产权的RAS肿瘤疫苗的项目,并通过与加州大学洛杉矶分校合作加速该项目的临床前药效学验证。公司目前的总部位于首都华盛顿近郊的马里兰,并已经在中国广州国际生物岛建立了研发中心。达冕公司在去年8月份完成了$235万美元的首轮种子融资。
达冕董事会董事长陆阳博士表示: “我们很高兴看到达冕生物的快速增长,并在这么短的时间内完成了两轮融资。最近Moderna和BioNtech对抗新冠病毒的mRNA疫苗取得的成功使我们坚信,用于开发新型治疗和疫苗的mRNA技术正在极具颠覆性地改变制药业的格局,也给我们带来了巨大的机遇。因此,达冕生物将继续培育和加强我们的全球创新努力,开发针对Covid-19和其他病毒感染的新疫苗,探索新的治疗方法与新肿瘤抗原方法或其他mRNA药物
百力司康B轮融资超4亿元,聚焦创新ADC
动脉网 2021-04-16
百力司康生物医药(杭州)有限公司(以下简称“百力司康”)宣布完成超过4亿元人民币B轮融资。本轮融资由高瓴创投领投,由Cormorant Asset Management、鼎珮集团(VMS Group)、和达生物医药基金共同参与投资,现有股东夏尔巴资本和东方富海持续加注。易凯资本在本次交易中担任百力司康的独家财务顾问。
本轮融资将用于BB-1701项目的国际多中心临床研究推进、产品线其他品种的临床前研究及注册申报和公司的研发生产基地建设。BB-1701是一种新型抗体偶联物(antibody-drug conjugate, ADC),目前正在进行中美联合一期临床研究,其毒素艾立布林(Eribulin)是从日本卫材引进的已获批上市的抗肿瘤小分子药,拥有临床验证的安全性和有效性。百力司康拥有该款ADC的开发和商业化的全球权益。公司第二款艾立布林ADC BB-1705项目预计将于今年中美双报。同时,公司也开发了具有自主知识产权的独特的定点偶联技术平台,正在积极推进梯度管线中多个best-in-class和first-in-class潜力产品的研发。
百力司康共同创始人、董事长及CEO魏紫萍博士表示:“我们非常荣幸能够获得生物医药领域顶尖投资机构的认可,感谢业界知名投资基金的鼎力支持以及现有股东的持续加持,也感谢我们落户的钱塘新区以及国内外合作伙伴在各方面的配合。希望在本轮融资的加速推动下,百力司康结合自主研发和外部合作高效地推进多个在研产品的开发和产业化,解决未满足的临床需求。”
Have You Heard Of The Chinese Biopharmaceutical Association (CBA)?
The Chinese Biopharmaceutical Association (CBA) is one of the largest Chinese American professional associations in the U.S. (www.cba-usa.org). It was founded in 1995 by Guo-Liang Yu, Ph.D., who today is the cofounder, chairman, and CEO of Apollomics, an oncology therapeutic discovery and development company (to be featured in an upcoming issue of Life Science Leader).


对抗疫情,CBA在行动
2020初始,新型肺炎疫情的进展速度超出了大家的预期。位于美国首都华盛顿的美国华人生物医药科技协会(CBA),本计划在大年初一举行一年一度的庆祝中国新年的盛装酒会。考虑到在大华府地区久负盛名的CBA Chinese New Year Gala规模大(近200人聚会),层次高(嘉宾包括联邦,州,郡和驻美使馆的官员和议员,各大公司主管以及著名艺术家),专业强(成员都是生物医药方面的专家),我们果断取消了这次酒会。这样的果断决定得到了全体嘉宾的由衷支持和感谢!

CBA 2020 Anti-COVID-19 Webinar Series-6
Global Pandemic Prevention and Start from Me Time: March 25th (Wednesday) 9:00-10:30pm (US Est. Time), March 26th (Thursday) 9:00-10:30am (Beijing Time) Language: ppt-English; Speaking-Chinese https://www.youtube.com/watch?v=xeorNeGP_jg&feature=emb_logo Everyone...

CBA 抗击新型冠状病毒知识系列讲座-2
CBA 抗击新型冠状病毒知识系列讲座-2 抗击新型冠状病毒创新药物的研发 演讲嘉宾:Dr. Patrick Lu, Sirnaomics Inc. Dr. Shoubai Chao, CanSino Biologics Inc. 点评嘉宾:Dr. Hang Lu https://www.youtube.com/watch?v=88Fq7hpIR4k&feature=emb_logo...
CBA抗击新冠病毒系列讲座精彩继续(二)
3月4日美国东部时间晚8:30,CBA第三期线上公益讲座如期举行。此次讲座围绕大家广泛关注的一个新药瑞德西韦(Remdesivir)展开。众所周知,瑞德西韦是吉利德(Gilead)制药研发的一款药物,在前期主要用于埃博拉(Ebola)病毒和中东呼吸综合征(MERS)等疾病的临床研究,在今年的COVID-19疫情中被快速用于临床试验,因此得到了全球医疗及制药相关领域的关注。此次讲座邀请了三位嘉宾,分别是周叶斌博士,Angela Zeng博士和张丹博士。...

CBA抗击新型冠状病毒知识系列讲座-1
CBA抗击新型冠状病毒知识系列讲座-1 新型冠状病毒解读和流行病防控战略演讲 嘉宾:Dr. Zhiming Zheng, NCI/NIH和Dr. Helen Schiltz, NIAID/NIH 庚子伊始,正值中国传统佳节春节来临之际,一场突如其来的“2019新型冠状病毒”疫情向我们无情袭来,不断蔓延的疫情更是牵动了无数CBA成员和朋友们的心,许多我们熟知CBA的骨干企业及个人,已经直接或间接地投入到了这场没有硝烟的战斗中! 抗疫情,CBA在行动!作为CBA抗疫行动的一部分,CBA Biomarker Study Group...

Dr. Guoping Zhao has accepted to be a CBA Scientific Advisor
Dr. Guoping Zhao has accepted to be a CBA Scientific Advisor Dear CBA members, supporters and friends, We have a very exciting news to share with you all! Dr. Guoping Zhao (赵国屏), a member of Chinese Academy of Sciences, has accepted the invitation to join our...

CBA 2019 Fall Symposium Ended with Great Success
The long-awaited CBA 2019 Fall Symposium just closed its 1 day event with great success at Johns Hopkins Montgomery County campus on the past Saturday, Nov. 23th, 2019. Hosted by CBA (Chinese Biopharmaceutical Association), this symposium has attracted over 200 attendees to this intensive and very informative event. Speakers invited by CBA came from areas crossing Maryland to Boston, from industry and government representatives, and also patient advocate. Hot topics in biopharmaceutical area were covered and heated discussions were raised between speakers and audience.

CBA’s 21st Annual Conference – in collaboration with MedImmune
CBA's 21st Annual Conference - in collaboration with MedImmune 2016年6月11日,来自中美生物医药领域的450余名企业家、专家、学者齐聚美国马里兰州盖瑟斯堡阿斯利康生物制药研发机构MedImmune总部会议中心,参加美国华人生物医药科技协会(CBA)第21届年会。此次年会以“中美生物制药:通过合作加速全球开发和商业化”为主题。阿斯利康执行副总裁兼MedImmune负责人Bahija...
亚盛医药杨大俊:中国创新药企业的征途是全球市场
2020年对于所有人都是异常艰辛与难忘的一年,我们见证历史,我们也改写历史。

Clinical Development and Innovation Forum 2021
临床开发与创新合作论坛 2021
Mar.10-11, 2021
Crown Plaza Shanghai NOAH Square, PR. China
Program Overview
China and Asia over the decades has been playing an increasingly important role in the context of clinical research activities due to the significant scientific investment by multinational companies as well as rapidly booming China domestic R&D programs. In past few years, the China regulatory agency has also issued several regulations and initiatives to accelerate the review and approval and improve the quality of new drugs and medical devices. With the joining of ICH in 2017, the government is continuing to improve the regulatory standards and systems to promote innovation, enhance the R&D ecosystem. However, the drug development is fraught with all kinds of challenges which needs innovation, efficiency and collaboration. In
return, the Clinical Development and Innovation Forum will gather leaders and scientists across pharma, biotech and academia for discussions and cases studies on the major topics including: Robust Clinical and Regulatory Strategy, Clinical Biomarkers and CDx, ImmunoOncology and Cell & Gene Therapies, Rare Disease, Data Sciences and Digital Innovation, AI and Machine Learning, China Domestic R&D Innovation, Clinical Operation and Patient Strategies etc, towards the Efficientand GCP-compliant Drug Development in the era of Precision Medicine.

Review
mRNA Vaccine—A Pioneer of COVID-19 Pandemic Termination
Miao Tan1,2, Li Li1,3, Tom Tang1, Haitao Yang1,4, Lei He1, Qiang Hu1,5*
1 Clinical and Regulatory Study Group. Chinese Biopharmaceutical Association, Potomac, MD, USA
2 Pfizer Inc., La Jolla, CA, USA
3 BLA Regulatory, LLC, Gaithersburg, MD. USA.
4 Shenzhen Institute for the Research, Sun Yat-sen University, Shenzhen, China
5 Hunter Medical LLC. Potomac, MD, USA
*Correspondence:
Qiang Hu, M.D. Executive Chairman, Hunter Medical LLC. Potomac, MD, USA
Email: patrickhumd@yahoo.com.
ABSTRACT
Vaccines are one of the major success stories of modern medicine. The development of vaccines progressed at a fairly slow rate until the last decade when new scientific discoveries and technologies led to innovative genebased vaccines. Gene-based vaccines are a completely new type of vaccine that are faster and cheaper to produce than traditional vaccines. mRNA vaccines use a different approach that takes advantage of processes
that are more efficient, cost-effective and safe. On the basis of these remarkable properties, mRNA vaccines quickly moved forward and within ten years were being used in some early clinical trials for infectious diseases and several types of cancer. The COVID-19 outbreak dramatically accelerated mRNA vaccines, moving them from development to authorized use in a record-setting ten months. In this review, we provide an overview of mRNA vaccine development and its application against the COVID-19 pandemic. As the first approved COVID vaccines, mRNA vaccines have been shown to be safe and mRNA technology will have a tremendous impact, not only on the pandemic, but also on the future treatment of many diseases [Am J Transl Med 2021.
5 (1): 13-24].
Two Immigrants, One Unique Plan For A Biopharma

HONG KONG, Feb 2, 2021 – (ACN Newswire) – The American Dream is why the United States is considered the “land of opportunity,” and why for centuries immigrants have flocked to our shores seeking their fortunes. Guo-Liang Yu, Ph.D., (China), and Sanjeev Redkar, Ph.D., (India), came to the U.S. in the 1980s. By anyone’s reasonable measure, both have achieved the American Dream. However, their dream as scientists remained at arm’s length. Their desire? Create a pharmaceutical company that leverages the best of what the U.S. and China have to offer toward discovering and developing new oncology therapeutics, while using the smallest drug development program possible to do so. This is the story of how the scientific careers of an intrapreneur (Redkar) and an entrepreneur (Yu) came together to cofound and co-lead Apollomics.
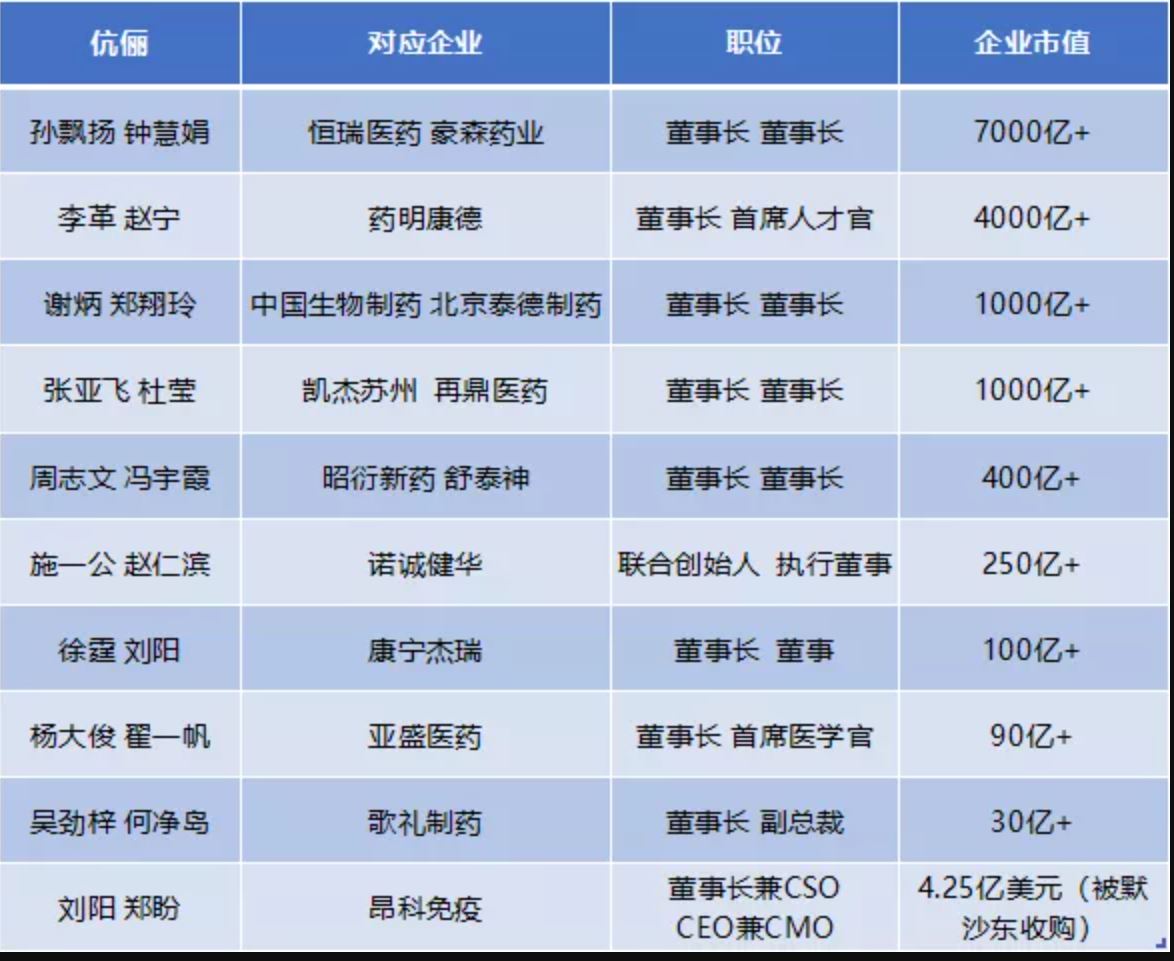
医药圈10对知名创业眷侣
康希诺生物-B(06185.HK)重组新型冠状病毒疫苗于墨西哥获得紧急使用授权











